
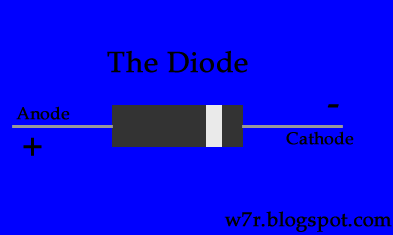
In practical systems, the primary electrode for stimulation is connected to a controlled voltage or current source, and an electrical return path is provided to circuit ground. Anodes are positively charged and attract negatively charged anions, being electron acceptors or sources of positive charge (oxidation reactions). It is an electron source and can donate electrons or accept positive charge in electrochemical reduction processes. A cathode is a negatively charged electrode that can attract positively charged cations. Stimulation electrodes are more analogous to electrolytic (Voltaic) cells. The events at the two poles of a battery (a Galvanic cell) are different from those at the pair of electrode connections during stimulation. Anode and cathode are functional and not structural terms ( Zoski, 2007).
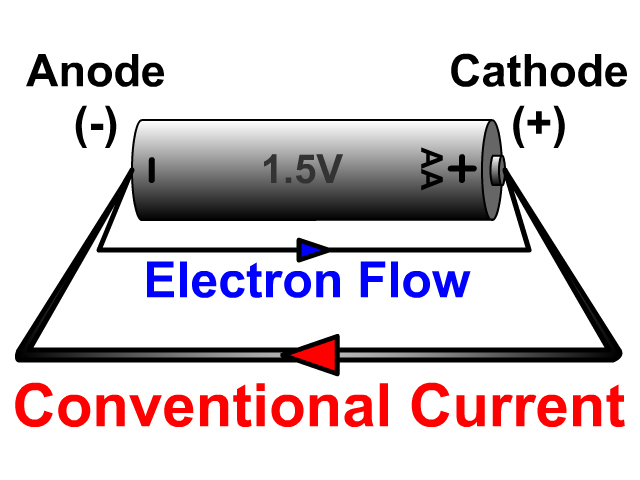
The terms anode (ana = up, hodos = path) and cathode (cata = down) were coined by Michael Faraday in the 1800s. The terminology of electrode connections can be confusing. Positive currents are thus defined to flow in a direction that is opposite to electron flow.

In external circuits, charge is transferred mainly by electrons that were designated to be with negative charge, after classical current directions had already been defined. Narendra Bhadra, in Implantable Neuroprostheses for Restoring Function, 2015 2.5.3 Anode and cathodeĮlectrical charges flow from points of higher to ones of lower potential.


 0 kommentar(er)
0 kommentar(er)
